Embark on a captivating journey with the Unit 2 Molar Mass Worksheet, a comprehensive resource designed to illuminate the intricacies of molar mass, a fundamental concept in chemistry. Through engaging explanations and practical examples, this worksheet unravels the mysteries of molar mass, empowering you to master this essential aspect of chemical calculations.
Delve into the world of molar mass, where you’ll discover its significance in determining the composition of compounds, predicting the products of reactions, and unraveling the secrets of chemical stoichiometry. Prepare to be captivated as we explore the diverse methods for determining molar mass and delve into the applications that make it an indispensable tool for chemists.
Definition and Importance of Molar Mass
Molar mass is a crucial concept in chemistry that represents the mass of one mole of a substance. It provides a bridge between the macroscopic and microscopic scales, allowing us to relate the amount of a substance to its mass.
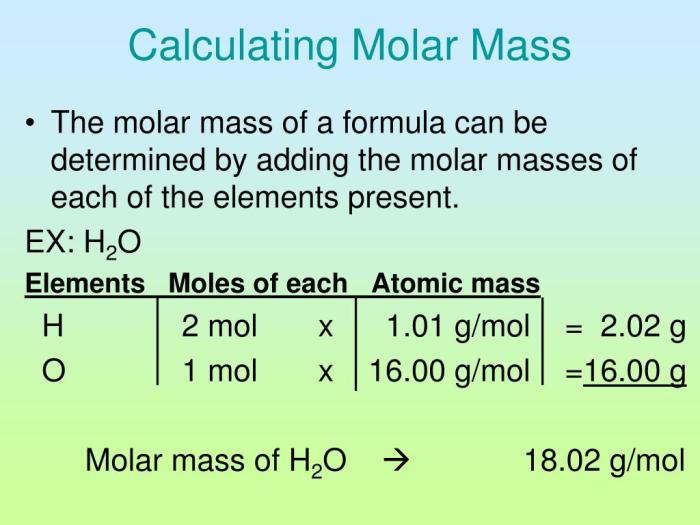
Molar mass is expressed in grams per mole (g/mol). It is calculated by adding the atomic masses of all the atoms in a molecule or formula unit. For example, the molar mass of water (H 2O) is 18.015 g/mol, which is the sum of the atomic masses of two hydrogen atoms (2 x 1.008 g/mol) and one oxygen atom (16.000 g/mol).
Applications of Molar Mass
Molar mass is a versatile tool used in various chemical calculations, including:
- Converting between mass and moles:Molar mass allows us to determine the number of moles of a substance present in a given mass or vice versa.
- Calculating the concentration of solutions:Molarity, a common measure of solution concentration, is expressed in moles of solute per liter of solution. Molar mass is used to convert mass concentrations (e.g., grams per liter) to molar concentrations.
- Determining empirical and molecular formulas:Molar mass plays a crucial role in determining the empirical and molecular formulas of compounds from experimental data.
Methods for Determining Molar Mass
Determining the molar mass of a substance is crucial for understanding its chemical properties and behavior. Various experimental methods can be employed to measure the molar mass accurately.
Mass Spectrometry
Mass spectrometry is a powerful technique that separates ions based on their mass-to-charge ratio. By analyzing the distribution of ions, the molar mass of the substance can be determined. Mass spectrometry offers high accuracy and can be used to analyze both organic and inorganic compounds.
The unit 2 molar mass worksheet is a valuable resource for students to practice calculating molar mass. If you’re looking for a break from the worksheet, why not check out missy d case départ lyrics ? Once you’re refreshed, you can come back to the molar mass worksheet and finish strong.
Elemental Analysis
Elemental analysis involves determining the elemental composition of a substance. By measuring the mass of each element present, the molar mass can be calculated. Elemental analysis is commonly used for organic compounds and provides reliable results.
Colligative Properties
Colligative properties, such as boiling point elevation and freezing point depression, are dependent on the number of particles present in a solution. By measuring these properties, the molar mass of the solute can be determined. Colligative property methods are simple and straightforward, but they may be less accurate than other techniques.
Vapor Density Method
The vapor density method measures the density of a gas relative to a reference gas, typically air. The molar mass of the gas can then be calculated using the ideal gas law. This method is particularly useful for gases and volatile liquids.
Advantages and Limitations
- Mass spectrometry:Advantages include high accuracy and versatility. Limitations include high cost and complex instrumentation.
- Elemental analysis:Advantages include reliability and simplicity. Limitations include the need for a large sample size and the inability to distinguish between isotopes.
- Colligative properties:Advantages include simplicity and low cost. Limitations include lower accuracy and the need for a known solvent.
- Vapor density method:Advantages include simplicity and applicability to gases. Limitations include the need for volatile substances and the potential for errors due to non-ideal gas behavior.
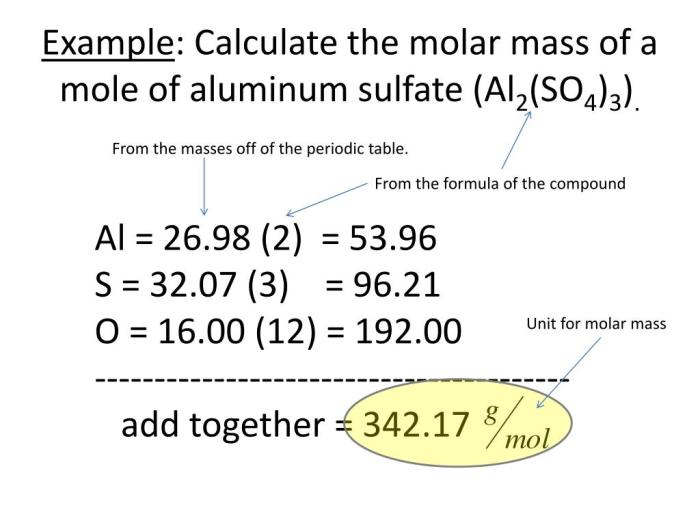
Molar Mass Calculations: Unit 2 Molar Mass Worksheet
Calculating the molar mass of a compound is essential for various chemical applications. It provides insights into the compound’s molecular weight and enables stoichiometric calculations.
Step-by-Step Guide to Molar Mass Calculations
- Write down the chemical formula of the compound.
- Locate the atomic mass of each element in the periodic table.
- Multiply the atomic mass of each element by the number of atoms of that element in the compound.
- Add the masses of all the elements to obtain the molar mass of the compound.
Examples of Molar Mass Calculations
| Compound | Chemical Formula | Molar Mass (g/mol) |
|---|---|---|
| Sodium chloride | NaCl | 58.44 |
| Water | H2O | 18.015 |
| Carbon dioxide | CO2 | 44.01 |
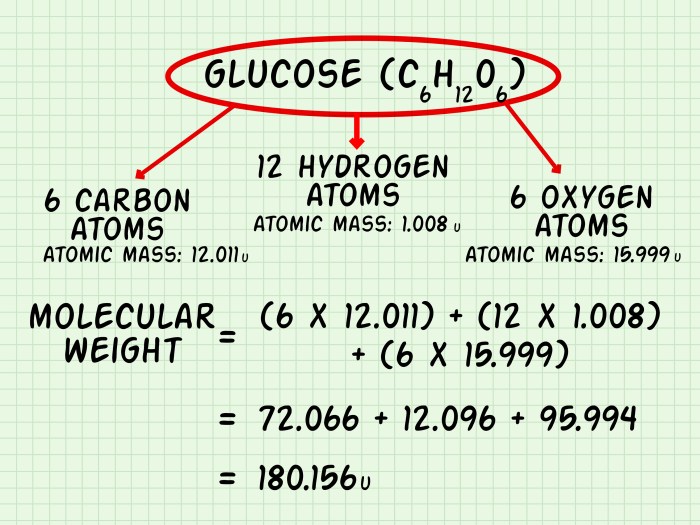
| Glucose | C6H12O6 | 180.16 |
| Sodium hydroxide | NaOH | 40.00 |
Applications of Molar Mass
Molar mass plays a pivotal role in various chemical calculations and applications. It serves as a bridge between the macroscopic and microscopic scales, enabling us to relate the mass of substances to their chemical composition.
Stoichiometric Calculations
Molar mass is indispensable in stoichiometric calculations, which involve determining the quantitative relationships between reactants and products in chemical reactions. By knowing the molar mass of each substance, we can convert between mass and moles, allowing us to calculate the number of moles of reactants required or products formed in a reaction.
Solution Chemistry
Molar mass is crucial in solution chemistry for preparing solutions with specific concentrations. It allows us to calculate the mass of solute needed to dissolve in a solvent to obtain a solution with a desired concentration. This is particularly important in analytical chemistry, where precise concentrations of solutions are required for accurate measurements.
Challenges and Errors in Molar Mass Determination

Determining molar mass with precision requires careful experimentation and analysis. Several factors can introduce errors into molar mass measurements, leading to inaccurate results. Understanding these challenges is crucial for minimizing errors and ensuring the accuracy of molar mass data.
Sources of Error
- Impurities:The presence of impurities in the sample can alter its mass and composition, affecting the calculated molar mass.
- Measurement Errors:Errors in measuring mass or volume can lead to inaccurate molar mass calculations. Using calibrated equipment and careful measurement techniques is essential.
- Method Limitations:Different methods for determining molar mass have inherent limitations. For example, freezing point depression measurements can be affected by solvent purity and solute dissociation.
Minimizing Errors, Unit 2 molar mass worksheet
To minimize errors in molar mass determination, several strategies can be employed:
- Purification:Purifying the sample before analysis removes impurities that could interfere with measurements.
- Multiple Measurements:Conducting multiple measurements and averaging the results reduces the impact of random errors.
- Calibration:Regularly calibrating equipment ensures accurate measurements.
- Appropriate Method Selection:Choosing the most suitable method for the sample and available resources minimizes method-specific errors.
Molar Mass and Chemical Reactions
Molar mass plays a crucial role in understanding and predicting the outcome of chemical reactions. It enables us to determine the quantitative relationships between reactants and products.
Balancing Chemical Equations
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side equals the number on the products’ side. Molar mass helps determine the correct stoichiometric coefficients (numbers in front of chemical formulas) to balance equations.
By converting masses to moles using molar mass, we can ensure that the moles of reactants and products are equal.
Predicting Reaction Products
Molar mass can also help predict the products of a reaction. By comparing the molar masses of reactants and products, we can infer the identity of the products. For example, if the molar mass of the products is significantly higher than that of the reactants, it suggests the formation of a more complex molecule.
Common Queries
What is molar mass?
Molar mass is the mass of one mole of a substance, which is the amount of the substance that contains Avogadro’s number of particles.
How do I calculate molar mass?
To calculate molar mass, you add up the atomic masses of all the atoms in the compound’s chemical formula.
What are the units of molar mass?
The units of molar mass are grams per mole (g/mol).