Periodic trends worksheet pdf answers provide a comprehensive overview of the fundamental principles governing the behavior of elements within the periodic table. These answers delve into the intricacies of atomic properties and their variation across different elements, offering a deeper understanding of chemical periodicity and its practical applications.
The periodic table organizes elements based on their atomic number, revealing distinct patterns and trends in their properties. Understanding these trends is crucial for predicting the behavior of elements and compounds, guiding the development of new materials, and unraveling the mysteries of chemical reactions.
1. Introduction
Periodic trends are regular and predictable variations in the properties of elements as we move across the periodic table. These trends provide valuable insights into the behavior and characteristics of elements, enabling us to understand and predict their chemical properties.
Periodicity is the concept that the properties of elements repeat in a predictable manner as we move from left to right across the periodic table and from top to bottom within groups. This periodicity is a result of the systematic increase in atomic number, which determines the number of protons and electrons in an atom.
2. Periodic Trends Worksheet PDF Answers
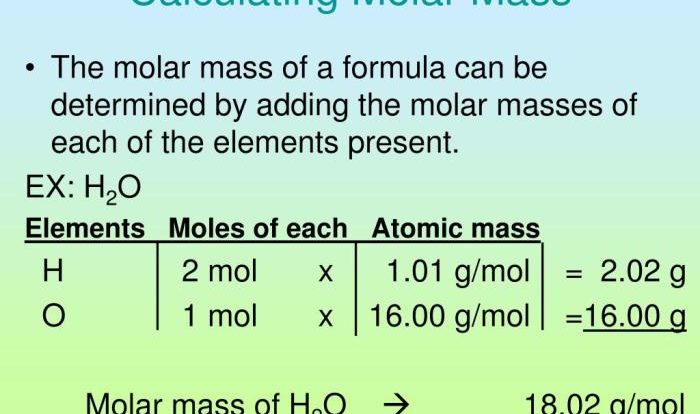
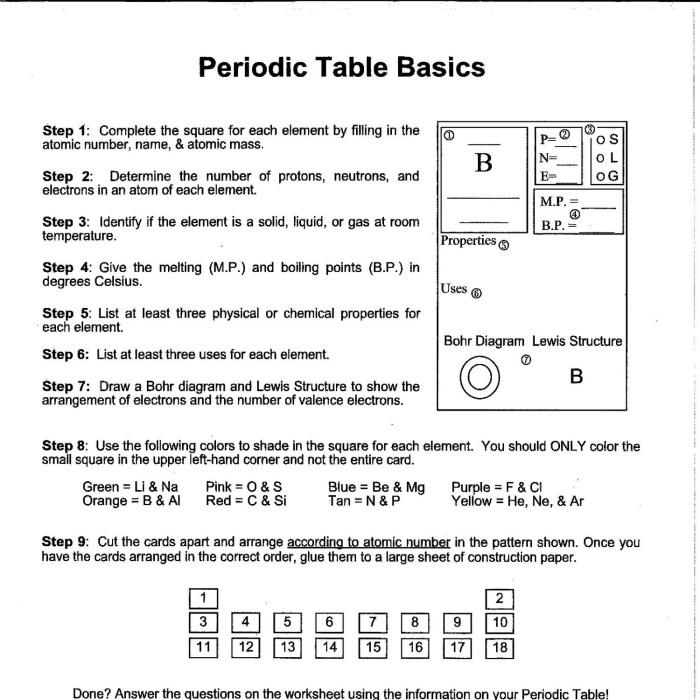
The attached PDF document contains a periodic trends worksheet with questions covering various aspects of periodic trends. The answers provided explain the underlying concepts and principles, highlighting the observed trends and patterns.
3. Examples of Periodic Trends: Periodic Trends Worksheet Pdf Answers
Atomic Radius, Periodic trends worksheet pdf answers
Atomic radius generally decreases from left to right across a period and increases from top to bottom within a group. This trend is due to the increasing nuclear charge (number of protons) across a period, which attracts the electrons more strongly, and the increasing number of electron shells down a group, which increases the distance between the nucleus and the outermost electrons.
Ionization Energy
Ionization energy is the energy required to remove an electron from an atom. It generally increases from left to right across a period and decreases from top to bottom within a group. This trend is due to the increasing nuclear charge across a period, which makes it more difficult to remove an electron, and the increasing distance between the nucleus and the outermost electrons down a group, which makes it easier to remove an electron.
Electronegativity
Electronegativity is the ability of an atom to attract electrons towards itself. It generally increases from left to right across a period and decreases from top to bottom within a group. This trend is due to the increasing nuclear charge across a period, which attracts the electrons more strongly, and the increasing number of electron shells down a group, which reduces the effective nuclear charge experienced by the outermost electrons.
Electron Affinity
Electron affinity is the energy change when an electron is added to an atom. It generally increases from left to right across a period and decreases from top to bottom within a group. This trend is due to the increasing nuclear charge across a period, which makes it more favorable to add an electron, and the increasing number of electron shells down a group, which makes it less favorable to add an electron.
4. Applications of Periodic Trends
Periodic trends have numerous practical applications in various fields, including chemistry, materials science, and medicine:
- Predicting the properties and behavior of elements and compounds.
- Designing and synthesizing new materials with desired properties.
- Understanding the reactivity and selectivity of chemical reactions.
- Developing new drugs and treatments in medicine.
- Optimizing industrial processes and technologies.
5. Limitations of Periodic Trends
While periodic trends provide valuable insights, there are some limitations and exceptions to these trends:
- Atomic size:Noble gases have larger atomic radii than expected due to their stable electron configurations.
- Ionization energy:Some elements, such as chromium and copper, have irregular ionization energies due to their stable half-filled or completely filled electron configurations.
- Electronegativity:Electronegativity values can vary depending on the chemical environment and bonding context.
6. Advanced Concepts in Periodic Trends
Effective Nuclear Charge
Effective nuclear charge is the net positive charge experienced by an electron in an atom. It considers the shielding effect of inner electrons, which reduces the attraction between the nucleus and the outermost electrons. Effective nuclear charge increases from left to right across a period and decreases from top to bottom within a group.
Shielding Effects
Shielding effects refer to the reduction in the attraction between the nucleus and the outermost electrons due to the presence of inner electrons. Shielding effects increase from left to right across a period and decrease from top to bottom within a group.
Detailed FAQs
What is the significance of periodic trends?
Periodic trends provide a framework for understanding the behavior of elements and predicting their properties based on their position within the periodic table.
How can periodic trends be applied in practical settings?
Periodic trends guide the design and synthesis of new materials with tailored properties, optimize chemical reactions, and enhance our understanding of various phenomena in chemistry and related fields.
What are the limitations of periodic trends?
While periodic trends generally hold true, exceptions may arise due to factors such as atomic size, electronic structure, and hybridization effects.